CBS17: "Mixing vaccines for boosters could produce higher antibody response, new study shows"
A new study from the National Institute of Health looked at mixing and matching each vaccine booster combination. When it comes to boosters, the study found it’s safe and effective to mix and match vaccines.
Drug Discovery News: Designing vaccines with reverse vaccinology
Most vaccine development begins with looking at the pathogen. Scientists pinpoint key residues needed for the virus to enter the body and develop vaccines that train the body to recognize signatures of the foreign invader. Kevin O'Neil Saunders, associate professor of surgery and director of research at the Duke Human Vaccine Institute at Duke University develops vaccines after analyzing the body’s immune response to a pathogen.
DHVI Immunology Quality Assessment (IQA) Program Receives Additional 7 year contract, Totaling 28 Consecutive Years for IQA
The Duke Human Vaccine Institute (DHVI) received a $16,218,499 (includes the base period and all options), seven-year (if all term options are exercised) contract to implement the Immunology Quality Assessment (IQA) Program from the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health (NIH), to provide a resource to evaluate and enhance the ability of U.S. and non-U.S. laboratories to participate in NIAID-funded and collaborative clinical studies.
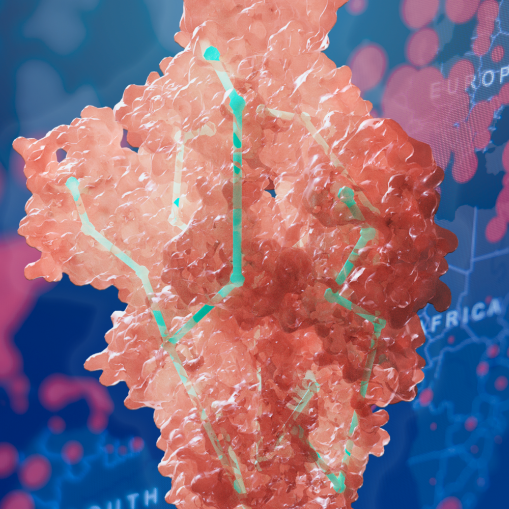
Duke study reveals mechanisms of increased infectivity, antibody resistance of SARS-CoV-2 variants
Combining structural biology and computation, a Duke-led team of researchers has identified how multiple mutations on the SARS-CoV-2 spike protein independently create variants that are more transmissible and potentially resistant to antibodies.
Antibody Disease Enhancement of COVID-19 Does Not Appear to Occur in Animal Models
In the fight against viruses, antibodies have the potential to either block infection or enable infection and make the disease worse, leading to concern about their use as a therapy for COVID-19.
Newly Identified Antibody Can Be Targeted by HIV Vaccines
A newly identified group of antibodies that binds to a coating of sugars on the outer shell of HIV is effective in neutralizing the virus and points to a novel vaccine approach that could also potentially be used against SARS-CoV-2 and fungal pathogens, researchers at the Duke Human Vaccine Institute report.
New Vaccine Blocks COVID-19 and Variants, Plus Other Coronaviruses
A potential new vaccine developed by members of the Duke Human Vaccine Institute has proven effective in protecting monkeys and mice from a variety of coronavirus infections -- including SARS-CoV-2 as well as the original SARS-CoV-1 and related bat coronaviruses that could potentially cause the next pandemic.
COVID-19 Laboratory Challenges and Learnings—One Year Later
This article is a follow up with Thad Gurley of the Duke Human Vaccine Institute (DHVI) Accessioning Unit, and Stuart Magoon of the City of Tacoma Environmental Services Laboratory (ESL) to gain their perspective on leading labs through a year of the pandemic.
Vaccines that can protect against many coronaviruses could prevent another pandemic
In 2017, three leading vaccine researchers submitted a grant application with an ambitious goal. At the time, no one had proved a vaccine could stop even a single beta coronavirus—the notorious viral group then known to include the lethal agents of severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS), as well as several causes of the common cold and many bat viruses. But these researchers wanted to develop a vaccine against them all.