In 2017, three leading vaccine researchers submitted a grant application with an ambitious goal. At the time, no one had proved a vaccine could stop even a single beta coronavirus—the notorious viral group then known to include the lethal agents of severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS), as well as several causes of the common cold and many bat viruses. But these researchers wanted to develop a vaccine against them all.
Grant reviewers at the National Institute of Allergy and Infectious Diseases (NIAID) deemed the plan “outstanding.” But they gave the proposal a low priority score, dooming its bid for funding. “The significance for developing a pan-coronavirus vaccine may not be high,” they wrote, apparently unconvinced that the viruses pose a global threat.
How things have changed.
As the world nears 3 million deaths from the latest coronavirus in the spotlight, SARS-CoV-2, NIAID and other funders have had a major change of heart. In November 2020, the agency solicited applications for “emergency awards” to pursue pancoronavirus vaccine development. And in March, the Coalition for Epidemic Preparedness Innovations (CEPI), an international nonprofit launched in 2017, announced it would spend up to $200 million on a new program to accelerate the creation of vaccines against beta coronaviruses, a family that now includes SARS-CoV-2.
The threat of another coronavirus pandemic now seems very real. Beyond bats, coronaviruses infect camels, birds, cats, horses, mink, pigs, rabbits, pangolins, and other animals from which they could jump into human populations with little to no immunity, as most researchers suspect SARS-CoV-2 did. “Chances are, in the next 10 to 50 years, we may have another outbreak like SARS-CoV-2,” says structural biologist Andrew Ward of Scripps Research, one of the scientists who submitted the 2017 proposal NIAID rejected.
The agency has not given out any of its new awards yet, but Ward’s lab is already pursuing a vaccine targeting a subset of beta coronaviruses. Some two dozen other research groups around the world have similar pancoronavirus vaccine projects underway. Their approaches include novel nanocages arrayed with viral particles, the cutting-edge messenger RNA (mRNA) technique at the heart of proven COVID-19 vaccines, and cocktails of inactivated viruses, the mainstay of past vaccines. A few teams have even published promising results from animal tests of early candidates.
No pancoronavirus vaccine has entered human trials, and how to evaluate a candidate’s protection against diseases that have not yet emerged remains a challenge. Nor is it clear how such a vaccine might be used. One possibility: keeping it in reserve until a new human threat emerges. “We might be able to prime everybody to get a basic level of immunity” against the emerging virus, buying time to make a more specific vaccine, Ward says.
Compared to flu and HIV, this is going to be relatively easy to accomplish.
Barney Graham, National Institute of Allergy and Infectious Diseases
Despite the many unknowns, the rapid success of vaccines against SARS-CoV-2 has sparked optimism. This coronavirus doesn’t seem particularly difficult to foil with a vaccine, which raises hopes that the immune system can be trained to outwit its relatives, too. Survivors of SARS years ago provide more encouragement: Some of their antibodies—an immune memory of that viral encounter—can also stymie the infectivity of SARS-CoV-2 in lab dishes.
NIAID’s Barney Graham, who helped develop Moderna’s mRNA COVID-19 vaccine, shares the optimism about pancoronavirus vaccines. “Compared to flu and HIV, this is going to be relatively easy to accomplish,” he predicts.
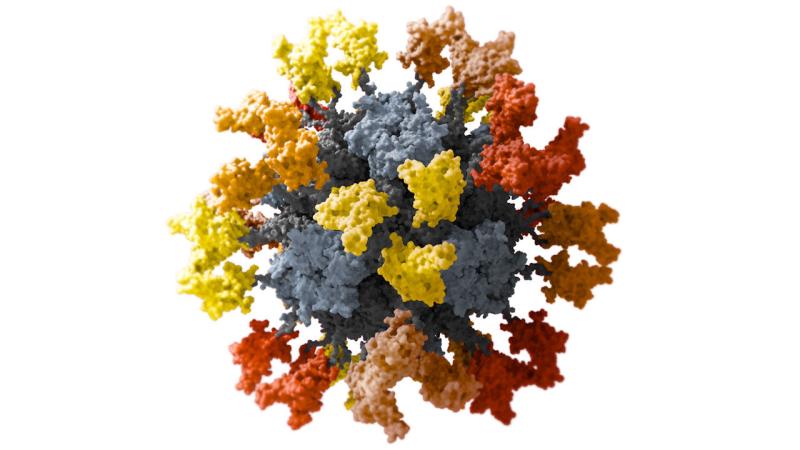
EARLIER THIS YEAR, Hannah Turner, a technician at Scripps Research who works with Ward, took a cold, hard look at a now infamous protein: SARS-CoV-2’s spike, which enables the virus to infect cells and is at the heart of several successful COVID-19 vaccines. All coronaviruses have these spikes, which create the distinctive crownlike appearance that earned them their name, and most pancoronavirus vaccine efforts aim to rouse an immune response to some part of the spike protein.
On this February morning, Turner mixes labmade copies of the SARS-CoV-2 spike with “broadly neutralizing” antibodies harvested from COVID-19 patients. These antibodies powerfully prevent multiple variants of SARS-CoV-2, as well as the original SARS virus, SARS-CoV, from infecting susceptible cells in test tube studies. Turner then freezes the spike-antibody mixtures with liquid nitrogen and places the resulting crystals in a $4 million microscope the size of three refrigerators. It begins bombarding the samples with up to 200 kilovolts of electrons to map the spike-antibody complexes at atomic resolution—an increasingly popular technique called cryo–electron microscopy (cryo-EM).
What resembles a telescope view of lunar landscapes unfolds across four monitors. Turner’s trained eye spots the crystallized spike proteins, clumped together in groups of three called trimers and studded with antibodies. She points out one of the fanlike structures. “It’s pretty cool,” she says. “This is what you want to see.”
The computers over the next few days will sort through 1100 different angles of her sample, migrating the best views into software that creates a gorgeous “final map” of spike with an attached antibody, at a resolution that approaches 3 angstroms, just a tad thicker than a strand of DNA. By creating similar portraits of spikes from many different coronaviruses with broadly neutralizing antibodies bound to them, Ward hopes to identify short segments of the protein—so-called epitopes—key to that binding for all the pathogens. Those epitopes, Ward believes, are the key to designing a vaccine that can trigger a broad immune attack on coronaviruses.
Finding the best shot
Aiming to prevent a future pandemic like COVID-19, scientists are looking for ways to immunize people against many, if not all, coronaviruses. Several strategies for these pancoronavirus vaccines focus on spike, the surface protein common to all members of the viral family.
An ideal pancoronavirus vaccine would protect us from all four of its genera—alpha, beta, gamma, and delta—but most researchers have more modest goals. “The further you go, the harder it gets” for a vaccine, says immunologist Dennis Burton of Scripps Research, who often collaborates with Ward.
Gamma and delta coronaviruses, mainly found in birds and pigs, are not known to infect humans, so vaccine developers have paid them little attention. There’s more interest in alpha coronaviruses because two cause colds in humans. But it’s the betas that attract the most effort, and in particular the sarbecoviruses, a subset that includes SARS-CoV-2 and SARS-CoV but not the more genetically distinct MERS and its relatives. Sarbecoviruses are Ward’s targets, and Burton is optimistic. “We already know you can get pretty damn good antibodies that work against both SARS-CoV and -2,” he says.
Ralph Baric of the University of North Carolina, Chapel Hill, who has studied coronaviruses for 35 years, sees this subgroup as the paramount threat. “That SARS-CoV-2 is here doesn’t mean that that’s going to provide any kind of serious protection against another sarbecovirus from coming out of the zoonotic reservoirs,” he says. And if a “SARS-CoV-3” jumped into a person infected with the current pandemic virus and created something more lethal by swapping genetic regions—and coronaviruses frequently recombine—that’s the making of a Hollywood horror film.
WARD AND OTHERS IN THIS nascent field are following the lead of vaccine successes, for diseases from polio to human papillomavirus, that depend on combining components from multiple forms of a pathogen—up to 23 forms of a sugar molecule in a shot against pneumococcal disease, for example—to increase breadth of protection. Although Ward is still at the earliest stages of the design process, trying to identify the conserved viral targets he wants a vaccine to hit, structural biologist Pamela Bjorkman at the California Institute of Technology is a few steps further along. Her team has recently evaluated candidate pancoronavirus vaccines in mice.
Bjorkman and co-workers chose a portion of spike from a range of beta coronaviruses: SARS-CoV and SARS-CoV-2, a virus isolated from a pangolin, and five bat viruses. They used each virus’ receptor-binding domain (RBD), the spike region that initiates an infection by docking onto angiotensin-converting enzyme 2 (ACE2), a protein on human cells. The RBD is the apparent target for most antibodies that thwart SARS-CoV-2.
Comparisons of the RNA genes encoding the spikes showed the top section of the RBD varied a great deal, but the lower part was conserved across the different viruses. So the investigators fashioned eight multimers—small proteins—from the conserved RNA sequences. Then, they fastened combinations of them onto the surface of a nanoparticle built from a bacterial protein to create various “mosaic” vaccines. In theory, a mosaic would produce antibodies that protected against the known viruses—and because the sequences are conserved, the vaccine might protect against distant relatives of those viruses as well.
Last year, Bjorkman and colleagues injected mice with some of their mosaic vaccines. They report in the 12 February issue of Science that in lab dishes, antibodies harvested from the mice powerfully neutralized the infectivity of a wide array of sarbecoviruses, including ones not used to make the vaccine.
Graham, who worked on pancoronavirus vaccines even before the pandemic, reasons that the whole trimer of spike might stimulate better or broader immune protection than just the RBDs. His team has taken spike trimers from SARS-CoV-2 and two beta coronaviruses that cause human colds and placed them in malleable nanocages, made from two different proteins developed by computational biologist Neil King of the University of Washington (UW), Seattle. The NIAID group is also using a second scaffolding: nanoparticles created from ferritin, a blood protein that normally sequesters and releases iron.
In a third strategy, Graham envisions giving people a series of vaccines, each one containing trimers from a different member of the beta genus, to create a broad defense against the viruses. “It’s what we do right now for influenza, basically, only it is occurring over a lifetime instead of more deliberately in a shorter period of time,” he says. (The flu shot contains surface proteins from three or four strains of that virus, and as it mutates, vaccinemakers change the ingredients annually.)
By arraying spike proteins on nanoparticles, vaccine developers aim to follow the style of upscale restaurants, where presentation matters nearly as much as the food on the plate. The goal is to cater to the tastes of B cells, the immune cells that pump out antibodies. The cells identify foreigners using Y-shaped proteins called immunoglobulins on their surfaces. (When secreted, the same proteins are antibodies.) B cells respond most strongly when each arm attaches to a different epitope. King says nanocages are ideal for presenting epitopes this way. “We can control spacing and geometry [of the viral pieces] in a much more precise way than anybody could before,” he says.
The resulting B cell cross-linking primes legions of the cells to spit out what researchers have dubbed “superantibodies” for their remarkably high potency. These superantibodies add to a vaccine’s breadth of protection because even when they aren’t a perfect match to a strain, they still retain some neutralizing activity.
Structural biologist Andrew Ward studies the nooks and crannies of a coronavirus spike protein for features common to spikes in other members of the virus family.
Baric’s group is exploring a different way to present diverse antigens. Rather than arranging them on scaffolds, the team uses mRNA coding for a chimeric protein that mixes and matches different parts of spike from distant human and bat sarbecovirus cousins. “Spike is really plastic, so it’s kind of a modular design,” Baric says. “You can move component parts around without any problem.” Four of these mRNA spike chimeras solidly protected mice from a variety of challenges with human and bat sarbecoviruses, Baric, Hayes, and colleagues reported in a bioRxiv preprint posted on 12 March.
Along with calculated strategies, luck can also aid the quest for a pancoronavirus vaccine. Barton Haynes and his team at Duke University, working with Baric’s group, recently designed a vaccine that contains a SARS-CoV-2 RBD in a ferritin nanoparticle. Intended as a booster dose for mRNA COVID-19 vaccines, it turned out to be far more versatile. In monkeys, it worked as intended against SARS-CoV-2. But much to the researchers’ surprise, antibodies taken from the vaccinated monkeys also neutralized SARS-CoV and two related bat coronaviruses in lab studies.
A clue to this surprisingly broad protection came when the team isolated an antibody from a person who had recovered from SARS many years ago. It, too, could neutralize a wide range of sarbecoviruses—and it turned out to bind tightly to the same RBD used in their COVID-19 vaccine booster. A structural analysis of the antibody bound to the RBD shows it latches onto the domain’s side, not the top region favored by most vaccine-induced neutralizing antibodies.
Because this antibody doesn’t interfere with ones that attach to the top, Haynes thinks a vaccine designed to trigger it and more common neutralizing antibodies could provide a one-two punch to multiple viruses. He expects clinical trials of this idea could begin within 6 months.
SOME GROUPS HAVE TURNED their sights far from the RBD, in molecular terms. Spike has both a head, which includes the RBD, and a stem—known as S2—that varies little between coronaviruses. “The S2 subunit is by far the most conserved portion of coronavirus spike,” says Jason McLellan, a structural biologist at the University of Texas, Austin, who co-authored the failed grant proposal with Ward.
After spike lands on a human cellular receptor, a coronavirus enzyme slices off the head, or S1, to expose the stem. That then, yes, spikes the cell and initiates the fusion that allows the virus to unload its genetic cargo. Immune responses against S2 can derail that key process, even though the stem isn’t always visible enough normally to initiate them.
A few years ago, McLellan developed a vaccine from the S2 of the MERS virus that protects mice from the virus as effectively as vaccines that feature the full spike. The antibodies produced by the vaccine also bind to, but do not neutralize, SARS-CoV, SARS-CoV-2, and a human cold beta coronavirus. McLellan’s lab is now conducting cryo-EM of antibody-stem conjugates, using S2 from SARS-CoV-2, to develop a vaccine for beta coronaviruses. “Our initial immunogens target all of S2, but we might want to refine that and target smaller portions,” McLellan says.
Like most developers of a pancoronavirus vaccine, McClellan is trying to rally antibody-producing B cells. A few groups, however, hope to also rouse the immune system’s other great army: T cells, which protect the body by destroying infected cells. On 18 May 2020 in Nature, Vir Biotechnology working with UW structural biologist David Veesler described an antibody, isolated from someone who had SARS in 2003, that neutralized infectivity of both SARS-CoV and SARS-CoV-2—but with help from T cells.
Chances are, in the next 10 to 50 years, we may have another outbreak like SARS-CoV-2.
Andrew Ward, Scripps Research
Although the antibody binds to the RBDs of spike of each virus, it does not block them from attaching to ACE2, cryo-EM showed. The groups instead found it may bind to the surface of immune warriors that educate T cells to destroy already infected cells. This “vaccinal effect” was first described more than 15 years ago in cancer research when scientists found that certain monoclonal antibodies can trigger killer T cells to eliminate tumors.
T cells are also central to the vaccine quest of Bette Korber, a computational biologist at Los Alamos National Laboratory. She designs algorithms to scour the genome sequences of beta coronaviruses, looking for regions of viral proteins that can trigger T cell responses, and that vary little among the different coronaviruses. Those conserved T cell epitopes, Korber says, might make a good vaccine.
She hopes to initially combine this T cell approach with a B cell strategy that would protect against all SARS-CoV-2 variants. It draws on an analysis of close to 1 million sequences of the virus now in databases to understand the “evolutionary space” of the pathogen—what changes could help it evade antibody responses and what mutations it cannot afford.
“You need to show the immune system what it needs to recognize to have breadth,” says Korber, who has applied similar techniques to designing vaccines for HIV, flu, and Ebola. Her collaborators plan to convert the sequences she selects into mRNA vaccines.
Finally, there’s an old-school approach to a pancoronavirus vaccine, one that should call into battle both B and T cells. NIAID’s veteran flu researchers Matthew Memoli and Jeffery Taubenberger want to combine inactivated versions of representative coronaviruses from the four known lineages in the beta genus. Vaccines based on the entire virus help the immune system take “multiple shots at the target,” Memoli explains, rather than focusing all the responses on spike or bits of it.
“Some antigens give you antibodies, some antigens may give you more T cell responses, some antigens may do both. Some antigens may be better at inducing mucosal immunity than systemic immunity,” he says. “The reality is that the best vaccine is going to deliver antigens that induce all of these responses.”
HOW CAN DEVELOPERS of pancoronavirus vaccines prove their shots protect against a hypothetical SARS-CoV-3? Baric highlights one hurdle: “You have to have a good panel of challenge viruses to actually begin to test these vaccines” in the lab. The U.S. government considers SARS-CoV, MERS, and many coronaviruses to be “select agents,” subjecting labs that handle them to greater restrictions. Baric notes that his lab is one of the few that has the biosecurity needed to grow and experiment with those coronaviruses.
Another regulation could ease the path. Created by the U.S. Food and Drug Administration in the wake of 9/11, when there was increased worry about engineered viruses or other biothreats, the “animal rule” says a therapy or vaccine can receive approval without an efficacy trial if the study cannot ethically or practically be done in humans. A pancoronavirus vaccine might establish its bona fides under that rule if it works in mice or monkey challenge studies against a variety of known coronaviruses, appears safe in humans, and is capable of triggering broadly neutralizing antibodies or other relevant immune responses in people.
If a pancoronavirus vaccine gets authorized, would countries create stockpiles to quickly extinguish an outbreak of a new virus? Or would they instead plan to rapidly start to produce the vaccine from its blueprint once that fresh threat is seen? Those are issues CEPI’s initiative will explore, but there’s a third, simpler option many in the field propose: using it in the current pandemic, as the ultimate booster shot to prevent potentially waning immunity and protect against the menacing new variants of SARS-CoV-2 that keep emerging.
Efforts have already begun to develop second generation COVID-19 vaccines that could protect against those variants. But Haynes says this is a game of “whack-a-mole” that has no end in sight. “You’re just waiting on the next variant to come up.” He and others instead propose that a pancoronavirus vaccine could do double duty. If it can handle other members of the coronavirus family tree, it should have no problem dealing with variations of SARS-CoV-2, which are minor in comparison.
“Over time, it may be that the boosting will be with a vaccine that is more broadly protective,” says Luciana Borio, who led a White House unit on medical and biodefense preparedness and now works for a venture capital firm, In-Q-Tel, whose portfolio includes biotech companies.
That might help end the current pandemic and forestall the next one. “A broadly protective vaccine has the goal of preventing a pandemic from happening,” Memoli says. “The issue we have is right now is if a completely new virus appears, we have nothing.”