DHVI Receives Contract to Manufacture Investigational Avian Flu Vaccines
The Duke Human Vaccine Institute received funding from the National Institute of Allergy and Infectious Diseases (NIAID), a branch of the National Institutes of Health, to manufacture H5N1 avian flu vaccines for use in clinical trials.
NIAID recently funded $7 million to Duke under the contract called the Collaborative Influenza Vaccine Innovation Centers (CIVICs), which NIAID set up to develop new influenza vaccines that induce long-lasting immunity and protect against more variants of the virus.
Duke Health Leads Its First Clinical Trial Testing a New Universal Flu Vaccine
Duke Health is leading its first clinical trial under a large federal initiative to develop a new-generation flu vaccine.
Duke Human Vaccine Institute Wins Contract to Produce Pan-Coronavirus Vaccine
The Duke Human Vaccine Institute has received a federal contract to manufacture a pan-coronavirus vaccine candidate that can be tested in a phase 1 clinical trial.
Awarded by the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health (NIH), the base period of the contract provides $11.2 million to support the program; additional provisions in the contract could increase the total funding up to $21.5 million if all option periods are exercised.
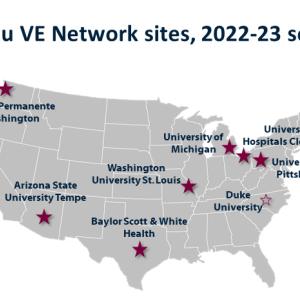
Duke Human Vaccine Institute to Serve as National Coordinating Center in Fight Against Influenza, COVID-19 and Respiratory Viruses
DHVI receives $4.9 million grant from CDC to serve seven U.S. sites