Biologic countermeasures, such as monoclonal antibodies (mAbs), are a rapid/effective means of controlling and containing outbreaks of emerging pathogens where no licensed therapeutic or vaccine is available. However, standard paradigms for their production and delivery limits their use as a first-line prophylactic or therapeutic, since the process for their identification, optimization and production can take months to years. The Defense Advanced Research Projects Agency (DARPA) has recently funded Duke as part of their Pandemic Prevention Platform (P3) to address these challenges.
The overall US Department of Defense DARPA program aims to develop an integrated technology platform that gives public health officials the capability to halt the spread of any viral disease outbreak within 60 days, before it can escalate to pandemic status. In contrast with state-of-the-art medical countermeasures, which typically take many months or even years to develop, produce, distribute, and administer, the envisioned P3 platform would cut response time to weeks and stay within the window of relevance for containing an outbreak.
The Duke DARPA Pandemic Prevention Platform (P3) team seeks to apply its experience, innovations, cutting-edge research portfolio, and in-house cGMP manufacturing capabilities to greatly expedite mAb countermeasures for future pandemics. The fully integrated platform will be a major advancement in rapid pandemic countermeasure development and will address the significant global challenge pandemic outbreaks have on both civilian and military populations.
The Duke DARPA P3 program (DARPA agreement number HR0011-17-2-0069) will combine world-class expertise in virology, immunology and CGMP manufacturing to create a fully integrated platform capable of responding to a viral pandemic. For this program, we are focusing on three main areas of innovation to development platform to be applied in future pandemics:
1. On-Demand Platform to Grow Virus: Develop methods to support viral propagation, so that virus can be used for downstream studies
2. System to Identify and Evolve Antibodies: Isolate neutralizing antibodies from convalescent PBMC and plasma, improve the antibody 100-fold in function by in vitro antibody evolution and transfer antibody sequence for production and delivery optimization/testing
3. Deliver Medical Countermeasures: Develop an mRNA-based medical countermeasure with optimal potency, half-life and ease of use in the field
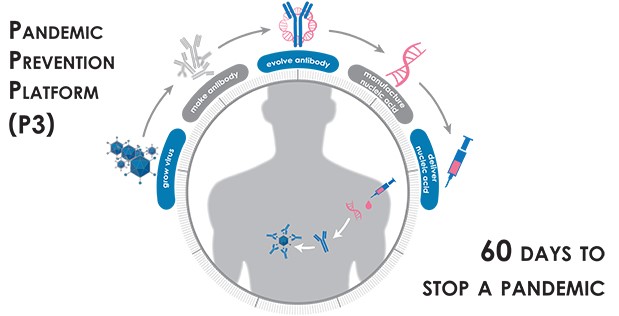
The DARPA Pandemic Prevention Platform (P3) program aims to develop an integrated platform that uses nucleic acid sequences to halt the spread of viral infections in sixty days or less. Using nucleic-acid-based technologies pioneered by DARPA as a foundation, the program now seeks to create an end-to-end platform by developing technologies to overcome remaining bottlenecks that hinder rapid response to pandemic threats. The three required technology areas cover growth of virus to support testing of treatments; rapid evolution of protective antibodies outside of the body; and safe and efficient delivery of nucleic-acid-based protective treatments. Figure and legend source.