Division of Structural Biology provides insight into SARS-CoV-2 spike mutation

Congratulations to the members of the Duke Human Vaccine Institute's Division of Structural Biology whose paper, "D614G mutation alters SARS-CoV-2 spike conformation and enhances protease cleavage at the S1/S2 junction" was recently published in Cell Reports.
In this study, led by Sophie Gobeil, PhD, the team investigates the effects of the SARS-CoV-2 spike D614G mutation, a single amino acid substitution that was identified early in the pandemic and quickly became the dominant variant worldwide. They show that the D614G mutation increases the “up” or ACE-2 receptor accessible state of the Receptor Binding Domain (RBD) and enhances furin protease cleavage of the spike, an essential step for the virus-host-cell fusion. Their structural analysis assigns a critical anchoring role to the SD2 domain, which is a key region that contains both the D614G mutation and the furin cleavage site. While the RBD and N Terminal Domains (NTD) in the S1 subunit are very mobile, the SD2 domain remains relatively immobile, separating large S1 subunit motions from the S2 subunit of the pre-fusion spike. Additionally, the team studied the “2P” mutations that were incorporated in the SARS-CoV-2 spike to prevent transition from its pre-fusion to post-fusion conformation. These mutations were designed based on prior structural studies with the MERS-CoV and SARS-CoV spikes and are included in the SARS-CoV-2 spike constructs that are part of current mRNA-based vaccines.
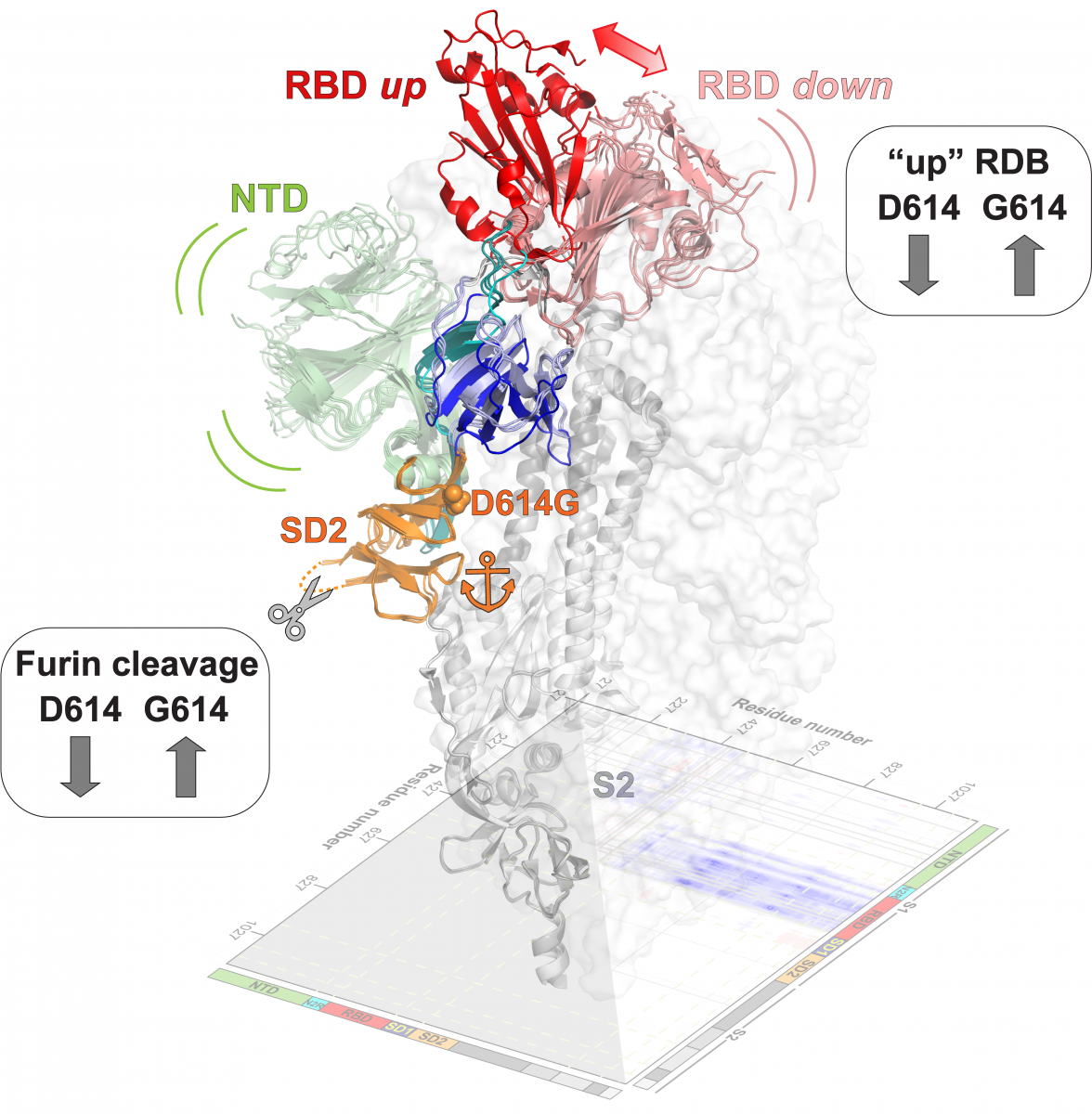
They find that, unlike in MERS and SARS, the 2P mutations have little effect on the structure, stability, and antigenicity of the SARS-CoV-2 spike, suggesting structural differences between the various CoV spikes that will be interesting to explore in future studies. Overall, the Division's findings reveal insights into the structure and biology of the SARS-CoV-2 spike and the D614G variant in particular, and provides atomic level information that should aid structure-guided immunogen design.
Read paper here.